On April 18, supported by People’s Daily Health Client as media partner and GeneIII Bio as a public welfare sponsor, the “GeneIII Ergothioneine Multi-Field Human Clinical Validation IIT Results Conference and Ergothioneine Academic and Application Exchange Meeting” was grandly held at the headquarters of the People’s Daily in Beijing.

The event gathered a wide range of industry and academic leaders, including Zhao Anping, Deputy Editor-in-Chief of People’s Daily Health Client; Professor Barry Halliwell, Senior Advisor for Academic Appointments and Excellence Research at the Office of the Provost, National University of Singapore (NUS), and Distinguished Professor at the Department of Biochemistry, Yong Loo Lin School of Medicine, NUS; Ding Wei, Founder and Chairman of GeneIII Bio; Dr. Wang Yang, Co-founder and Chief Scientist of GeneIII Bio; Dr. Yang Qinghua of Temasek Life Sciences Laboratory; Tao Chunlei, Chairman of Anhui Wanbang Pharmaceutical; Wang Dahong, Founder of Shuzheng Kangxun Market Intelligence Think Tank; Guo Hui, Deputy Director of the Zhonglian Liver Health Promotion Center; along with leaders from top hospitals, pharmaceutical enterprises, industry think tanks, and over 100 beauty and health KOLs (Key Opinion Leaders), to witness the release of GeneIII’s ergothioneine human clinical IIT results in the fields of liver and eye health.
As a strategic emerging industry at the national level, synthetic biology is rapidly reshaping the health and wellness sector. GeneIII Bio, leveraging its globally leading synthetic biology technology for ergothioneine production, has successfully achieved the first multi-field human clinical IIT validation results.

01
Technological Breakthroughs Driving Industry Innovation
Ergothioneine Clinical IIT Validation Leading a New Trend in Health Consumption

At the start of the conference, Zhao Anping, Deputy Editor-in-Chief of People’s Daily Health Client, delivered an opening speech, stating:
“Synthetic biology and the health industry are both nationally strategic sectors. As a strategic emerging industry, synthetic biology is a key engine for upgrading biomedicine and health industries. I call on academia, industry, and research sectors to deepen collaborative innovation.”

Ergothioneine is a natural sulfur-containing amino acid and a powerful antioxidant, historically dubbed “the golden antioxidant” due to its high production cost.
“To make ergothioneine accessible to the general public instead of remaining a luxury, we achieved leaps in purity and production capacity while reducing costs by 90% through synthetic biology technology,” shared Ding Wei, Founder and Chairman of GeneIII Bio, discussing the team’s three years of intensive efforts.
“Ergothioneine’s protective effects for the eyes and liver have moved from ‘theoretical possibility’ to ‘clinical proof’. Over the past year, two human clinical studies targeting dry eye and liver function have yielded significant results, confirming its efficacy in eye and liver health fields,” Ding added.

Professor Barry Halliwell, Senior Advisor for Academic Appointments and Excellence Research at NUS and Distinguished Professor at the Department of Biochemistry, Yong Loo Lin School of Medicine, widely recognized as the “Father of the Free Radical Theory of Aging,” delivered a keynote speech titled “Recent Advances in Ergothioneine Research.”
He provided a detailed overview of current research progress, study designs, and experimental outcomes.
Professor Halliwell emphasized that ergothioneine, with its unique molecular mechanisms, is emerging as a multi-target breakthrough ingredient in the anti-aging field. It offers cross-organ protection, particularly in skin and neurodegenerative disease prevention.
As humans cannot synthesize ergothioneine naturally and endogenous levels sharply decline with age, exogenous supplementation has become a scientifically necessary means of maintaining cellular antioxidant functions.
He noted that ergothioneine’s multi-dimensional mechanisms provide an irreplaceable biotechnology solution for developing anti-aging products.
In skin, ergothioneine penetrates the epidermal layers to the dermis, reducing collagen degradation and improving photoaging. In the nervous system, it crosses the blood-brain barrier to protect neurons, offering potential in early-stage prevention of Alzheimer’s disease and other neurodegenerative disorders.
Tao Chunlei, Chairman of Anhui Wanbang Pharmaceutical, speaking from a CRO (Contract Research Organization) perspective, pointed out that the breakthroughs in clinical results for liver and eye applications are not the end: four additional functional areas are currently undergoing clinical research.
GeneIII Bio maintains a “data doesn’t lie” principle from molecular mechanism exploration to clinical protocol design, and such a “science-first, evidence-driven” R&D philosophy is the core competitiveness for China’s health food sector.

Wang Dahong, Founder of Shuzheng Kangxun Market Intelligence Think Tank, Secretary-General of the Market Work Committee of the China Health Care Association, and Chairman of the Health Products Committee of the China OTC Drug Association, called for greater attention to the hidden and widespread issues of liver health, highlighting ergothioneine’s value in intervening in metabolic liver injury.
He noted that liver disease incidence among Chinese residents has surged over the past decade, with related public awareness also intensifying.
Balancing oxidative stress is critical for liver protection. Research confirms that ergothioneine, with its high stability and bioavailability in the human body, effectively scavenges excess free radicals, reduces oxidative stress, and protects cell membranes and DNA integrity.
Moreover, ergothioneine shows great potential in preventing and treating various chronic diseases such as cardiovascular diseases, neurodegenerative diseases, and diabetes.

Wang Yanxu, General Manager of Early Data, analyzed 2024 dietary supplement consumption trends from a nutritional health market and ergothioneine industry perspective.
She shared that the dietary supplement market in 2024 reached $125.5 billion in sales, growing 12.0% year-on-year. The ergothioneine ingredient category saw an explosive 1071.5% year-on-year growth.
In the liver health sector, ergothioneine, as a newly added compound in functional formulations, grew by an astonishing 8199.4%, becoming one of the fastest-growing ingredients in the liver health market.
In the eye health field, ergothioneine also began gaining traction and growing rapidly starting in 2024.
The rapid growth of ergothioneine across multiple sectors is mainly driven by cross-border e-commerce channels, with more than half of sales coming from Tmall Global.
Consumer feedback highlights its recognized efficacy in liver protection, improving complexion, and enhancing sleep quality.
Data indicate that the nutrition and health market has entered a “dual-engine” era driven by “innovative dosage forms + functional ingredients,” and ergothioneine is becoming a major new growth driver.
02
Clinical Evidence Anchors Multi-Track Eye and Liver Health Protection
Accelerating the Industrialization of Functional Formulations

At 3 PM, the much-anticipated release of GeneIII’s original ergothioneine multi-field IIT human clinical study results took place. Dr. Jiang Jiang, Principal Investigator of the ergothioneine eye discomfort IIT human clinical trial, Director and Chief Physician of the West District of Hefei First People’s Hospital, and Standing Member of the Visual Rehabilitation Committee of the Anhui Rehabilitation Medical Association, presented the GeneIII ergothioneine eye wash clinical study results.
Dr. Jiang explained that according to literature research, ergothioneine can alleviate ocular oxidative stress and help in antibacterial defense by reducing nitrosative stress and maintaining lactoferrin activity. The eye has the highest ergothioneine concentration among body organs (at molar levels), where it plays key antioxidant, anti-inflammatory, and antibacterial roles. As the body ages and the eyes remain exposed to environmental factors, oxidative damage increases, and ergothioneine consumption leads to weakened antioxidant and antibacterial abilities. Major blinding diseases like cataracts, glaucoma, and age-related macular degeneration are closely linked to oxidative stress and decreased antioxidant defenses.
Clinical trial results showed that after using GeneIII’s ergothioneine eye wash, participants exhibited significant decreases in Dry Eye Questionnaire scores, Ocular Surface Disease Index (OSDI) scores, and Visual Fatigue scores. Tear film break-up time in dry eye patients improved by an average of 27.74% compared to baseline. [1]

At the same time, Ms. Shao Feng, General Manager of Anhui Linka Medical Technology Co., Ltd., head of the clinical CRO for the ergothioneine liver function IIT trial, reported on GeneIII’s ergothioneine capsule human clinical research:
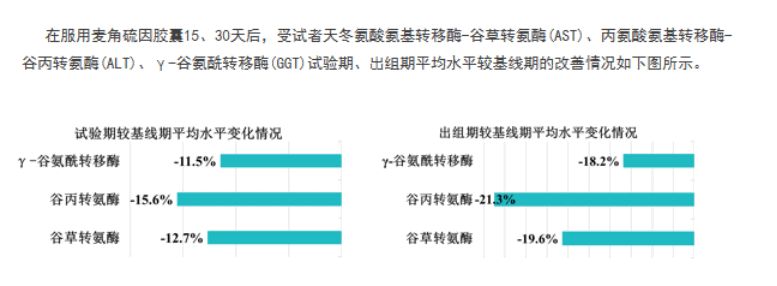
Clinical data from randomized double-blind trials conducted in tertiary hospitals showed that after 30 days of continuous supplementation with GeneIII ergothioneine capsules, serum ALT and AST levels decreased significantly by 21.3% and 19.6%, respectively, compared to the control group—meeting pharmaceutical-grade efficacy standards. Furthermore, participants’ daytime dysfunction scores (reflecting fatigue and energy levels) improved by 51.56% from baseline. [2]
03
Synthetic Biology Reshapes the Health Industry
Academia-Industry Roundtable Builds an Innovation Ecosystem

As a key practice in reshaping the synthetic biology industrial ecosystem, the event featured an “Industrial Resonance Roundtable Forum.” In the “Eye Health Innovation Ecosystem” panel, Mr. Zhu Kuo, Partner at Jifu Asia, moderated discussions with Prof. Barry Halliwell (NUS), Prof. Zhang Yu (Dean, School of Pharmacy, Shenyang Pharmaceutical University), Mr. Ding Wei (Founder and Chairman of GeneIII), Mr. Liu Yun (Founder of Huayi Capital), and Dr. Jiang Jiang.
Prof. Halliwell highlighted ergothioneine’s blood-brain barrier penetration and irreversible antioxidant properties, noting its potential to revolutionize treatments for neurodegenerative diseases like Alzheimer’s. Dr. Jiang emphasized the urgent clinical need for dry eye solutions in China, with over 360 million patients, and praised GeneIII’s eye wash for restoring ocular microenvironment homeostasis—filling a clinical gap.

In the “New Era of Liver Health Protection” forum, Mr. Kang Shuangming (Founder, Hongming Tech Consulting) moderated discussions with Mr. Wang Dahong (Founder, Shuzheng Market Intelligence), Dr. Wang Yang (Co-founder and Chief Scientist of GeneIII), Dr. Guo Hui (Deputy Director, China Liver Health Promotion Center), and Dr. Yang Qinghua (Temasek Life Sciences Laboratory, Singapore).
Dr. Wang Yang shared GeneIII’s innovations in strain engineering, highlighting how their use of gene editing, AI, and biosensors transformed traditional fermentation tanks from “black boxes” to “white boxes,” enabling real-time monitoring of biochemical reactions.
“In Chinese philosophy, balance between yin and yang is the way of the Dao,” Dr. Wang said. “Managing the balance between oxidation and antioxidation during reactions is about approaching natural laws.”
04
Establishment of the GeneIII International Expert Advisory Board
Advancing Ergothioneine Clinical Research Translation
At 5 PM, the formation of the “GeneIII Ergothioneine Clinical Research Expert Advisory Board” was announced, climaxing the event. Participants included GeneIII founders Ding Wei and Dr. Wang Yang, Prof. Barry Halliwell, Prof. Zhang Yu, Dr. Jiang Jiang, Mr. Wang Dahong, Ms. Tao Chunlei (Founder and Chairwoman of Anhui Wanbang Pharmaceuticals), Mr. Liu Yun, Dr. Yang Qinghua, and Dr. Kang Shuangming. The Advisory Board will focus on mechanisms research, clinical expansion, and standard-setting to promote Chinese synthetic biology achievements internationally.

This event at the People’s Daily headquarters not only showcased leading scientific achievements but also highlighted deep collaboration between national media and cutting-edge biotech companies.

GeneIII is setting a benchmark in synthetic biology, combining clinical validation, international expert collaboration, and full industry chain layout to establish a closed-loop “research-clinical-industrial” development model. From the People’s Daily stage, GeneIII is powerfully broadcasting China’s biotech capabilities to the world.

Moving forward, GeneIII plans to accelerate multicenter clinical trials of ergothioneine in anti-aging and liver health, leveraging the expertise of the world’s first Ergothioneine Clinical Research Expert Advisory Board, and continuing to break technical barriers in synthetic biological manufacturing—bringing “Made in China” biotech solutions to global healthcare.
References:
- [1] Data from Ergothioneine Eye Wash Human Clinical IIT Report, Registration Number: ChiCTR2400090987
- [2] Data from Ergothioneine Capsule Human Clinical IIT Report, Registration Number: ChiCTR2400093739


